Monitoring Barrier Properties of MDCK Cell Layers During Depletion of Cell Cholesterol by Methyl-ß-Cyclodextrin
Plasma membrane lipids such as cholesterol play an important role in regulating the barrier function of epithelial cell layers. Lipids contribute to the structure, assembly and function of tight junctions and thereby have an effect on the selective permeability of the paracellular pathway. A common in vitro approach to study the underlying mechanisms is to selectively alter the lipid composition of the plasma membrane and then employ analytical techniques to determine possible effects on the barrier properties. In this study Madin-Darby canine kidney (MDCK) cells grown on porous membrane inserts were exposed to different concentrations of methyl-ß-cyclodextrin (MBCD). This agent serves as a cholesterol binding reagent and thereby allows one to selectively lower the content of cellular cholesterol.
The cellZscope® provides valuable information on the barrier properties and the differentiation stage of cell layers by measuring their transepithelial electrical resistance (TER) and capacitance (Ccl). In contrast to other analytical techniques these electrical measurements can be performed marker-free on living cells, leaving them fully viable for further experiments. This makes the cellZscope the ideal tool for monitoring cells while they grow confluent and differentiate. Once the cells have reached a steady-state subsequent experiments can be performed while the cellZscope continues to measure the electrical parameters characterizing the barrier function and the stage of differentiation.
Monitoring the cell layer and junction formation
MDCK-II cells were seeded (density 5×105 cells/cm2) in serum-containing medium on the porous polycarbonate membrane (pore density 1×108/cm2, pore size 0.4µm) of standard cell culture inserts (Corning, Transwell®, #3401). Cells were allowed to settle and attach to the membrane for 10 hours. Then the formation of a differentiated cell monolayer with established cell-cell junctions was monitored with the cellZscope by measuring the electrical resistance and capacitance. As shown in the diagram the time course was followed for 60 hours with automatic recording of data points for each of the 24 wells every hour.
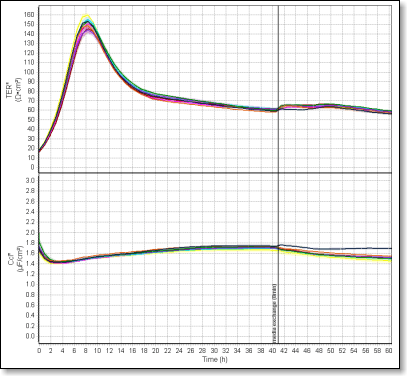
Time course of the transepithelial electrical resistance and capacitance of MDCK-II cells. The cellZscope allows continuous data recording while the cell cultures remain in the incubator.
The initial steep increase in TER clearly marks the onset of junction formation. This process was completed after 8 hours when TER reached a maximum with full establishment of a tight junction network. The subsequent decrease in TER can be attributed to an increasing cell number per surface area, i.e. the total perimeter length of cell-cell contacts connected in parallel increases. As a consequence the total resistance of the network, i.e. TER decreases. Finally, after approximately 40 hours TER converged to a steady state level. At this time point the medium was replaced with serum-free medium and the cell layers allowed to adapt for further 20 hours.
Monitoring the barrier function and cell differentiation
The change to serum-free medium was performed in order to minimize cholesterol content in the medium prior to MBCD treatment. Thus MBCD kept its depletion potential for selectively lowering cell cholesterol levels. Then different concentrations of MBCD were added to the top compartment of the wells, i.e. to the apical side of the cell layers. The response of the MDCK cells was analyzed by monitoring TER and Ccl with the cellZscope.
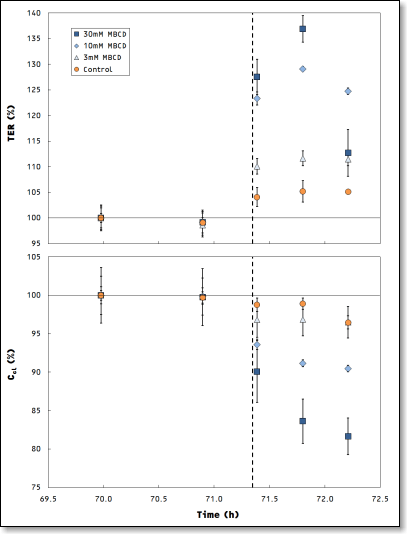
Time courses of TER and Ccl exhibit a dose dependent response of the MDCK-II cells to MBCD treatment (dashed line). Data points represent the averaged mean and error bars the standard deviation of three wells.
The observed initial increase in TER is well know from literature and different interpretations of the underlying biochemical mechanisms were suggested [1, 2]. The results obtained with the cellZscope contribute to ongoing investigations in this field by providing the cell layers’ capacitance Ccl as an independent readout parameter. This type of complemental data recorded simultaneously with TER reveals another dose dependent change in the cell layers’ properties: it directly indicates morphological changes in the plasma membrane.
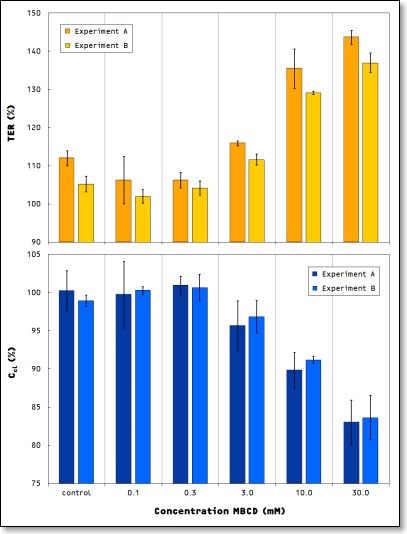
Results of two independent experiments showing the dose dependent response in TER and Ccl of MDCK-II cells to cholesterol depletion after 30 min. of exposure to MBCD.
The observed reduction in the total membrane capacitance caused by exposure to MBCD is exactly in line with independent investigations based on confocal microscopy [3]: these studies revealed that the depletion of cell cholesterol leads to the retraction of surface microvilli and microridges. Consequently, Ccl shows a significant, dose dependent decrease, since the cell layer’s electrical capacitance directly depends on the morphology of the plasma membrane. In particular, a retraction of protrusions such as microvilli and microridges causes a decrease of the effective surface area and thereby leads to a decrease of the electrical capacitance. The cellZscope is tailored to detect and monitor such changes in the plasma membrane during the full time course of an experiment.
nanoAnalytics thanks K. Hardes and K. Riehemann (Westfälische Wilhelms-Universität Münster) and J. Wegener (Universität Regensburg) for their comprehensive support of this study.
[1] Stankewich, M., Francis, S.A., Vu, Q.U., Schneeberger, E.E., Lynch, R.D., Alterations in Cell Cholesterol Content Modulate Ca2+-Induced Tight Junction Assembly by MDCK Cells. Lipids 31, 817 (1996).
[2] Francis, S.A., Kelly, J.M., McCormack, J., Rogers, R.A., Lai, J., Schneeberger, E.E., Lynch, R.D., Rapid reduction of MDCK cell cholesterol by methyl-ß-cyclodextrin alters steady state transepithelial electrical resistance. Eur. J. Cell. Biol. 78, 473 (1999).
[3] Colarusso, P., Spring, K.R., Reticulated Lipid Probe Fluorescence Reveals MDCK Cell Apical Membrane Topography. Biophys. J. 82, 752 (2002).